| LC can be (and
has been) carried out using a glass tube hand-packed with
powder through which a solvent is allowed to gravity-flow.
So why do we need all the complicated high-tech equipment?
There are many aspects to the answer: Speed. A single analysis by "Classical" LC can take anywhere from 2 to 12 hours to carry out. HPLC allows an equivalent analysis to be done in 2 to 12 minutes. Reproducibility. A classical column must be freshly packed for each analysis, increasing the chance of errors. A single HPLC column can be used for hundreds or thousands of samples. Quantitation. Classical LC was (and still is) an excellent technique for sample preparation or purification, but requires additional collection and analysis steps to be useful as a tool for quantitative analysis. HPLC uses on-line detectors that provide quantitative information during the course of the separation. Sensitivity. Classical LC used relatively large columns and correspondingly large volumes of mobile phase solvents. The resulting dilution limited the sensitivity of the technique. HPLC uses miniaturized columns to keep sample dilution to a minimum.
|
||
|
||
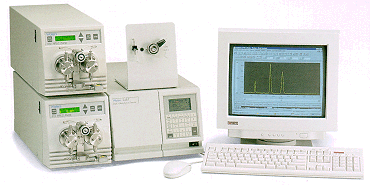
| The end result is an HPLC
system composed of six basic modules (reservoir, pump,
injector, column, detector, and data system) connected by
appropriate tubing and fittings. Although systems from
different manufacturers can look quite different
externally (see below for some examples) they all follow
the same basic pattern illustrated in the figure at the
right.
|
 |
|