| Let's begin by
examining the stationary phase (column packing) used for
reversed-phase separations. This column packing consists
of very small particles that are squeezed together very
tightly inside the column. If the column packing material
is magnified so that we can actually see the individual
particles, they are usually spherical and look like very
small tennis balls. To the naked eye, however, column
packing looks more or less like baby powder; the
individual particles are much too small to see clearly.
|
||
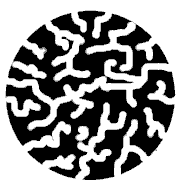
| Let's imagine that we can
slice a stationary phase particle in half (like cutting
an apple) and look at the resulting cross-section. We
would then see something like the diagram at the right.
The solid black parts represent the material of which the
particle is made, while the white regions represent holes
or pores that criss-cross the particle in all directions
(this figure is not drawn to scale; the actual particles
are much larger relative to the pores than we have shown
here). Most of the particle is composed of silica - a
glass-like material that provides the underlying
structure. The surface of the silica (both on the outside
of the particle and within the pores) is covered with a
layer of organic molecules; these molecules are
chemically bonded to the silica surface so as to form a
tight, chemically resistant coating. This organic layer
is often referred to as the "bonded phase".
|
A particle of column packing is honeycombed by pores. Most of the surface area is inside these pores (this drawing is *not* to scale; typical pore diameters are on the order of 1-5% of the particle diameter).
|
|
| Now
let's magnify part of a pore wall still further, as shown
at the right. We can more clearly see the structure of
this surface bonded layer of organic (the "bonded
phase"). The organic layer consists of threads or
alkyl chains attached to the silica surface. Sample
molecules stick to these alkyl chains and are thereby
retained inside the packing particle, thus causing
retention of the sample.
|
Magnified cross-section of a pore, showing a layer of alkyl chains bonded to the silica surface
|
|
| As sample
molecules are swept through the column by the flowing
mobile phase, they move in and out of the pores.
Compounds that "stick" to the bonded phase will
be slowed down and leave the column later than compounds
that do not stick. Different sample components (analytes)
will bind to the stationary phase more or less tightly,
depending on the chemical nature of the compound. As we
discussed in Section 1, sample retention depends on the
interaction of the analyte with the mobile phase on the
one hand and with the stationary phase (column packing)
on the other hand. Generally, compounds that are more
similar to the mobile phase will interact with it more
strongly and therefore will be washed through the column
quickly. Compounds that are more similar to the bonded
phase will tend to stay on the packing particle and thus
wash through the column more slowly.
|
||
For reversed-phase chromatography, packings are
grouped according to the chemisty of the bonded phase
chains. The usual packings include the following:
There are other column packings that are used for reversed-phase LC less often (cyano, diphenyl, etc.).
|
||
| While it is
important to specify the column-packing type as described
above, this is not a sufficient description to ensure
that a column will work for your assay. Because of
differences in manufacturing chemistry, a C18 column from
Waters is not the same as a C18 column from Agilent or
from Supelco or any other supplier. In many cases, a
given supplier will have multiple (different) columns
that fit the same general descripion. For example, Waters
sells C18 columns called Microbondapak, Novapak, and
Symmetry, and they are not the same. Agilent sells C8 or
C18 columns under different names such as Zorbax, Zorbax
Rx, Zorbax Stablebond, or Zorbax Eclipse, each of which
is made for a different use.
|
||
| The size of the particles
of column packing is very important, and this should
always be specified. Most columns come with particles of
5-micron (mm), although both smaller and larger
particles are also used. The column length and width
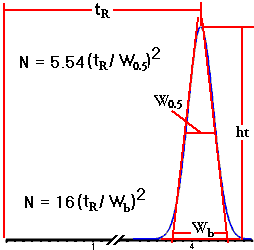
should also be noted. We have seen that a good separation requires narrow peaks in the chromatogram. However it is hard to describe a column in terms of peak width because early peaks are narrower than later peaks (assuming constant conditions). The column "plate number" (N) is a way to avoid this problem, because the plate number is fairly constant for all the peaks in a particular chromatogram. The plate number can be measured from the width and retention time as shown here.
|
Plate number (N) is calculated from retention time (tR) and peak width (either baseline width, wb, or width-at-half-height, w0.5).
|
|
| The value of N
for a column varies with many factors: column length,
particle size, mobile phase flow rate, how well the
column was made, and how long the column has been in use.
The usual 10- or 15-cm long columns packed with 3- or 5-micron
particles have values of N of approximately 10,000 plates
when new and when operated under ideal conditions. Column
plate numbers for the separation of "real"
samples are generally between 2,000 and 5,000.
|
||
| It is
important to check the plate number of a column when it
is first received and installed on your LC system. The
manufacturer will provide a test chromatogram with
the column showing the separation of a particular sample
(usually a mixture of compounds) with a particular set of
conditions (flow rate, mobile phase, temperature, etc.)
specified. You should be able to get the same separation
reported in the test chromatogram, and the plate numbers
for different peaks should be within 10 or 20% of the
reported values. The test chromatogram should be saved,
as it is useful in checking for certain column problems
at a later time.
|
||
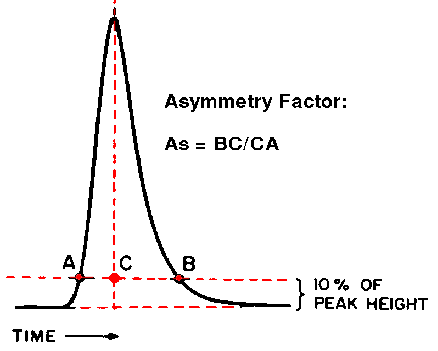
| Now that we've covered the
plate number, let's discuss peak asymmetry. Good LC peaks
should be perfectly symmetrical, but there is often a
certain amount of tailing, as seen in the peak at the
right. This particular peak actually tails rather badly. Peak tailing or asymmetry is bad for many reasons; it makes it hard to measure peak size, and it is a symptom of other column problems. We can measure peak asymmetry in one of two ways as shown here. The Tailing Factor, measured at 5% of the peak height, is largely used in the pharmaceutical industry. The Asymmetry Factor measured at 10% of the peak height is most often used in non-pharmaceutical analyses. In most cases, the Asymmetry Factor and Tailing Factor will be roughly the same (although rarely exactly equal). Values should normally fall between 1.0 and 1.5 for a new column and the conditions of the test chromatogram. Less symmetrical peaks are often observed under actual running conditions with "real" samples, but any increase in peak tailing is a symptom of a problem that should be fixed.
|
|
|
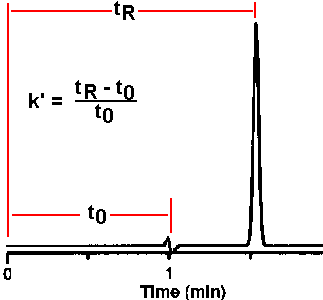
| A good separation also
requires reasonable values of retention. While retention
time is a direct measurement of retention, it has the
disadvantage of being affected by mobile phase flow rate
as well as system chemistry (increasing the flow rate
causes a proportionate decrease in retention time). A more useful measurement is the capacity factor, or k'. This is calculated from the retention time of a peak and the dead time of the column as shown at the right. This takes advantage of the fact that both dead time and retention time are affected the same way by changes in flow rate or column dimensions; these effects cancel so that the capacity factor is unaffected by flow rate or column dimensions; it is controlled entirely by the temperature and by the chemical characteristics of both the stationary phase and the mobile phase. The dead time of the system is unaffected by changes in mobile phase composition or column chemistry.
|
Capacity factor (k') is calculated from the retention time of a peak (tR) and the dead time of the system (t0).
|
|
| If a sample has two or
more peaks, we can also calculate a retention ratio (a) for
any pair of peaks. This is simply the ratio of capacity
factors (k' of the second peak divided by k' of the first
peak). It gives information about the selectivity of the
chromatographic system. Like the capacity factors that
comprise it, the retention ratio is unaffected by flow
rate or column dimensions; it is controlled entirely by
the temperature and by the chemical characteristics of
the stationary phase and the mobile phase.
|
The retention ratio (a) is calculated from the capacity factors of two adjacent peaks. It is always greater than or equal to 1.0.
|
|