| So far, we
have been considering only HPLC separations in which the
mobile phase composition remains constant during the
course of the chromatogram -- so-called "isocratic"
separations. Although most separations you will encounter
are isocratic, there are times when no single mobile
phase composition gives usable results for all the peaks
in the separation. The separation of polynuclear aromatic
hydrocarbons shown below provides a good example. When
the mobile phase composition is adjusted to give
reasonable retention for the early peaks, the late peaks
have an excessively long retention time. If we decrease
the water content in the mobile phase to make it stronger,
the late peaks are reasonably retained, but now the early
peaks are poorly resolved.
|
||
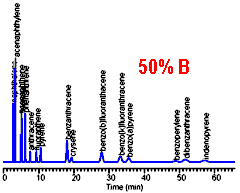
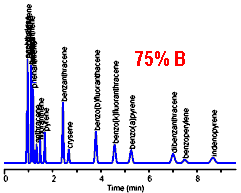
The chromatogram on the left provides a good separation for the early peaks, but excessive retention time for the late peaks. Increasing the mobile phase strength (%B) results in reasonable retention for the late peaks, but loss of resolution for the early peaks. There is no single isocratic composition that allows for good chromatography of all the peaks in this sample.
|
||
| We can deal with the
problem by starting the separation with a weak (high-water
content) mobile phase, and then gradually increase the
mobile phase strength (decrease the water content) in
order to speed the elution of the more strongly retained
components. This technique of increasing mobile phase
strength during the chromatogram is called "gradient"
HPLC.
|
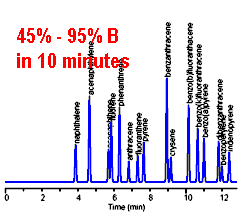
Gradient HPLC -- changing the mobile phase composition from 45% B to 95% over 10 minutes provides good separation of all the peaks in a single chromatogram.
|
|
| If you carry
out HPLC separations of "biopolymers" such as
peptides, proteins, or nucleic acids, you will find that
gradient separations are the rule rather than the
exception, because these large molecules typically have a
wide range of retention within a single sample.
|
||
A gradient
HPLC system is necessarily more complex than an isocratic
system, because it must incorporate some means of
accurately changing the mobile phase composition during
the run. Two basic types of pumping system are used:
Chromatographers continue to debate the trade-offs and relative merits of these two approaches; good (and bad!) designs have been based on both types.
|
||
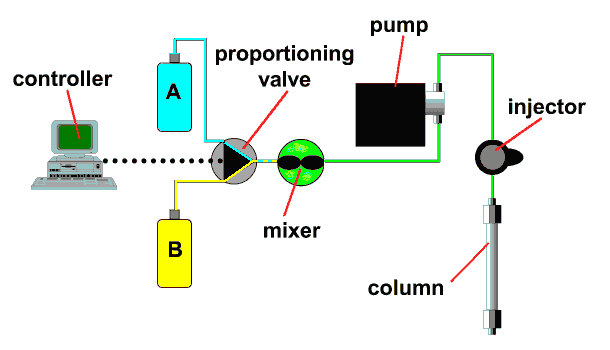
A high-pressure mixing (two-pump) system blends the output of two high-pressure pumps on the downstream side of the pumps to control the mobile phase composition.
|
A low-pressure mixing (one-pump) system uses a proportioning valve mounted upstream of a single high-pressure pump to control the mobile phase composition.
|
|
| By convention,
the weak solvent in a gradient separation is referred to
as the "A" solvent, the strong solvent is
referred to as the "B" solvent. A typical
linear gradient separation is defined by specifying the A
and the B solvent, specifying the initial and final %B
and the time over which the change occurs.
|
||
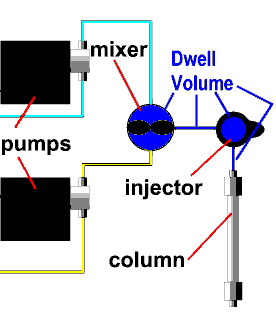
| An important (and under-appreciated)
characteristic of a gradient system is its dwell volume (also
called the "gradient delay volume"). This is
the volume of liquid in the system between the point
where the gradient is formed (usually at the
proportioning valve or mixing chamber inlet) and the
point where it enters the column. This imposes a de-facto
isocratic hold or delay at the beginning of each gradient
chromatogram. If a method was originally developed on an
instrument with a small dwell volume and then run on a
system with a larger dwell volume (for example), the
result will be an offset in retention times; in some
cases, selectivity may also change and accuracy of the
results may be compromised.
|
The dwell volume in a gradient system is the total volume between the point where the mobile phase is blended and the column inlet. In a high-pressure mixing system as shown here, it includes the mixer, transfer tubing, and swept volume in the injector or autosampler.
|
|