| Fortunately, we usually
know what compounds to expect in a particular sample. For
example, if we are analyzing tablets for aspirin, we
assume that aspirin will be present in each tablet. Or if
we are trying to measure the purity of some compound
"X" by determining its concentration in the
sample, we assume that compound X is present. When we
want to measure the concentration of a compound or
compounds in a sample by means of HPLC, we first
determine the retention time of the pure compound as
sample. If we want to measure compound A, we inject pure
A into our LC system, using the same conditions that we
use to measure A in different samples. In this case we
might obtain the chromatogram at the left.
|
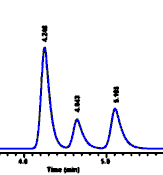
Chromatogram of pure standard drug "A"
|
|
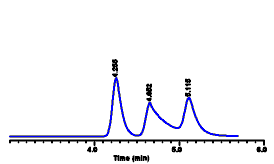
| We see that compound A has
a retention time of 4.25 minutes. Now if we want to find
out if some mixture or sample contains this compound, we
inject the sample and obtain the chromatogram at the
right. In this case we see that the first peak also has a
retention time of 4.25 minutes, and we therefore assume
that peak #1 in the sample is compound A. We might also
want to measure compounds B and C in the sample, so we
inject them too: By matching up the various retention
times, we see that peak #2 in our sample is compound B (retention
time of 4.64 minutes) and peak #3 is compound C (retention
time of 5.1 minutes). If our retention times don't match
closely for the pure compound and for the sample, then
probably we have made a mistake - the compound is not
present in the sample.
|
Chromatogram of sample mixture. The first peak has the same retention time as that of the drug "A" standard (above). The second and third peaks have the same retention times as standards of drugs "B" and "C" (not shown)
|
|
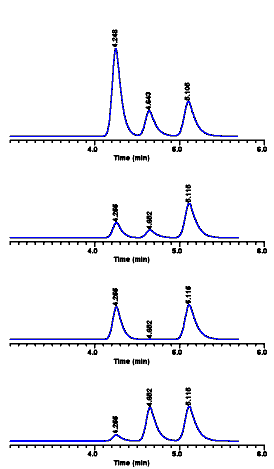
| When we run different
samples containing compounds A, B, and C as above, we
generally find that the size of the different peaks
changes from run to run. This means that there are
different amounts of A, B, and C present. However as long
as A, B, or C is contained in the sample, its retention
time should not change. That is, the retention time of a
compound is normally not affected by peak size or the
amount of the compound present in the sample. If compound
A (retention time equals 4.25 minutes) is absent from a
given sample, then no peak will be found at 4.25 minutes
- we can then report that the concentration of A in the
sample is zero.
|
|
|
| In other cases, we may
find extra peaks in the chromatogram besides those due to
compounds we are analyzing for. This means that there are
additional compounds in the sample, compounds we may not
have expected to be present. The presence of unexpected
bands in the chromatogram should be pointed out to the
person for whom the LC analysis is intended - sometimes
this affects the use that will be made of the sample
analysis.
|
|
|
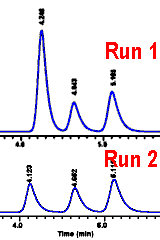
| Often we find
that retention times for different compounds remain
fairly constant from day to day. That is, compound A
gives the same retention time (4.25 minutes) in every LC
run. In other cases we may see small variations in
retention time from one day to the next: This may be
normal, and it usually is when the changes in retention
time are only a few hundredths of a minute - and when
retention changes in the same direction for all bands.
For larger changes in retention time, as shown below at
the right, the pure compounds should be reinjected to
confirm that the sample bands are the same compounds we
are analyzing for. In any case, pure compounds should be
injected at least once a day to confirm sample bands.
Never rely on yesterday's injection of a pure compound to
identify today's sample peak. Also keep in mind that
changes in retention time can be a symptom of some
problem with the analysis procedure. This is especially
true when just one band shows a change in retention time,
while other bands do not change as we see here.
|
||
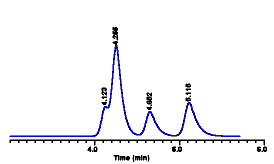
| Here peak #1 (compound A)
shows changed retention time of 4.1 minutes in the second
run. This could be the result of some interfering
compound that is overlapping peak #1, which could cause
serious error in the reported value of compound A. In
this case pure compound A should be injected to confirm
its retention time. If we find that something is wrong with this run, the concentration of compound A in this sample should not be reported.
|
|
|
| It is also possible to see
changes in peak shape within the chromatogram. Here peak
#2 has developed a tail and is no longer a symmetrical
peak. This suggests that some interfering compound is
overlapping peak #2 in the chromatogram, and again, the
concentration of compound B in this sample should not be
reported.
|
|
|