| Now we are
ready to talk about the actual process of calculating
results from an LC analysis. The chromatogram gives us
two important pieces of information for each band:
retention time and peak size (either area or peak height).
The retention time tells us what compound the band
represents. The peak size can be converted into the
concentration of that compound in the sample that was
injected into the LC system.
|
|||||||||||
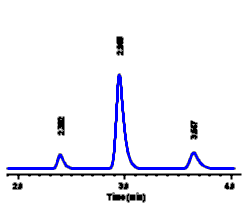
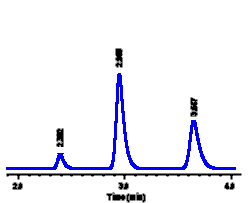
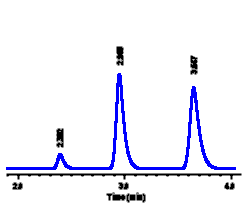
| For example,
we might make up solutions of methyl paraben (a food
preservative) at three different concentrations and
inject each of these into our LC system: In each case a
peak is seen at the same retention time (3.65 minutes),
as expected for different samples of methyl paraben.
However the size of the peak in each sample is different
- a higher concentration of methyl paraben is seen to
yield a larger peak in each case. In order to calculate
the concentration of methyl paraben in a sample, we need
to be able to measure peak size.
|
|||||||||||
|
|||||||||||
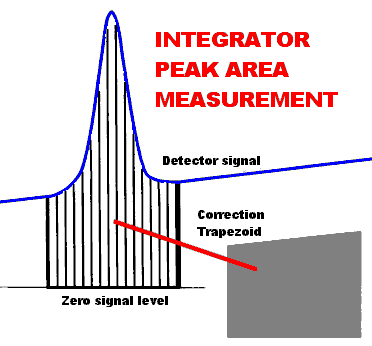
| The size of the peak is
most frequently determined as peak area. We seldom
measure peak areas manually - it is just too tedious and
imprecise. Instead we rely on the data system. Data
systems work by slicing up the peak as shown on the right.
Then the height of each slice is measured and all the
slice-heights are added together to give the area.
Finally, a "correction trapezoid" is computed
to account for the offset between the baseline and
electronic zero; the area of this trapezoid is subtracted
from the raw area to give the actual area under the peak.
The data system can also measure peak height by using the
height of the tallest slice. Integrators do the best job of measuring peak height or area when the peak is well resolved and the baseline is relatively level.
|
|
||||||||||
| In the absence
of a data system, we can measure the peak height manually
with a ruler, simply recording the distance from the
baseline to the top of the peak. The units of peak height
will cancel out when we calculate the concentration of
the compound giving the peak. That is, whether we measure
peak height in centimeters, millimeters, or % of full
scale, you can forget the units (just measure ALL peaks
with the same units).
|
|||||||||||
Now our series of methyl paraben samples yield the following information, once our integrator has done its job (or we have measured the individual peak heights manually): The next step is to use these data to obtain a "calibration plot" (sometimes also called a "calibration curve") so that we can analyze any sample for methyl paraben.
|
Calibration data for methyl paraben showing retention times (tR), peak area, and name for each peak in the calibration runs |
||||||||||
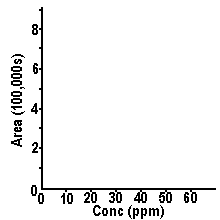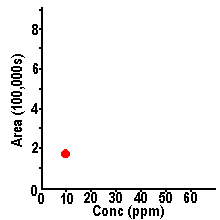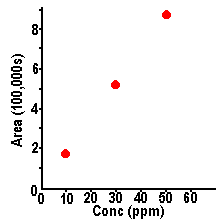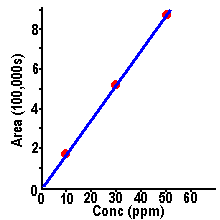
| Calibration curves are
obtained by plotting peak size on the y-axis versus
sample concentration on the x-axis - for a series of
samples with known concentrations. Using the data above
for the 10, 30, and 50 mg/L samples of methyl paraben, we
can generate the plot shown here. We see a straight line
through the data points, and the line passes through the
zero-zero point of the graph - or through the origin.
|
|
||||||||||
| Calibration
plots like these are called "linear" and yield the best analytical
results. They also permit the simplest approach to sample
analysis - as we will see in the following section. The
straight-line plot shows that peak size increases
proportionately with sample concentration. If we double
the concentration, we also double peak size.
|
|||||||||||