| So far, we
have have discussed various manipulations of the sample (weighing,
dilution, etc.) and related calculations. Often two or
more of these operations are combined into a single assay
procedure. Here we will look at the calculation of a
final assay result in such cases.
|
||||||||||||||||||
We can
summarize our approach to various problems as follows:
|
||||||||||||||||||
In our first
example, consider the analysis of a liquid formulation
for phthalic acid using a single-point calibration. We
begin by preparing our calibrator; i.e., weighing out
phthalic acid into a volumetric flask and filling to mark
with solvent. The procedure for this method calls for 0.5
grams of phthalic acid to be dissolved in 50 mL of
deionized water:
|
||||||||||||||||||
Our analytical
procedure calls for first diluting the 1 mL of the liquid
sample to 25 mL, followed by injecting the diluted sample
into the LC system. The dilution factor for the sample is
calculated from:
|
||||||||||||||||||
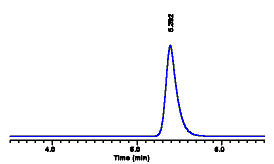
Next we run our calibrator
and obtain a peak at 5.4 minutes with an area of 20,000
counts. Because we are using a single-point calibration,
we must calculate the sensitivity factor:
|
Chromatogram of phthalic acid calibrator solution (10 mg/mL). The area of the peak at 5.4 minutes was measured as 20,000 counts.
|
|||||||||||||||||
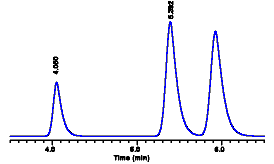
When we run the diluted
sample, we obtain a peak at 5.4 minutes with an area of
30,000 counts. Now we can calculate the concentration of
diluted sample:
|
Chromatogram of sample containing phthalic acid. The area of the peak at 5.4 mnutes was measured at 30,000 counts. |
|||||||||||||||||
What is the
concentration of phthalic acid in the original (undiluted)
sample? We obtain this from the concentration of the
dilute sample and the dilution factor:
|
||||||||||||||||||
In a second example, consider the analysis of a solid sample for dimethylchickenwire (DMCW). We begin by weighing out a certain quantity of DMCW and dissolving it in a certain volume of solvent. Let's assume the following values for calibrator and samples 1 and 2:
|
||||||||||||||||||
Now, three chromatograms
are run with the following results:
|
|
|||||||||||||||||
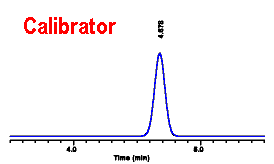
We first
calculate the sensitivity factor for the DMCW peak in the
calibrator:
|
||||||||||||||||||
Next we
calculate the concentration of DMCW in diluted sample #1:
The concentration of the total sample in the solution is 42.0 mg / mL, so the fraction of DMCS in the original solid sample #1 is:
|
||||||||||||||||||
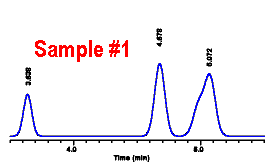
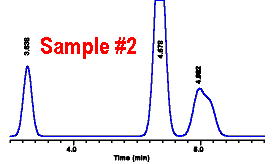
Sample #2 was
off-scale, so it must be diluted and rerun. Five mL of
sample was diluted into a 25 mL flask. The dilution
factor is:
|
||||||||||||||||||
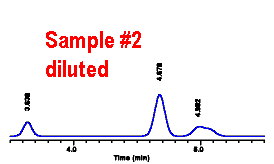
| The diluted sample was
reinjected. The resulting peak at 4.68 minutes had a peak
area of 36,000. The concentration of DMCW in this diluted sample of #2 was then:
|
|
|||||||||||||||||
The
concentration of DMCW in the original (undiluted) sample
is given by the concentration in the diluted sample and
the dilution factor:
The concentration of the total sample in the solution is 39.80 mg / mL, so the fraction of DMCS in the original solid sample #1 is:
|
||||||||||||||||||