I would like to know how does helium acts to degass mobile phase and what are the recomendations for more efficient degassing.
![]()
![]()
![]()
![]()
![]() By Anonymous on Tuesday, July 6, 2004 - 07:05 am:
By Anonymous on Tuesday, July 6, 2004 - 07:05 am:
Helium gas is inert gas and lighter gas. when you purge your mobile phase with helium gas it will replaces the dissolved gases. Helium gas after replacing other gas helium itself escape from mobile phase and your mobile phase get degass.
![]()
![]()
![]()
![]()
![]() By tom jupille on Tuesday, July 6, 2004 - 10:35 am:
By tom jupille on Tuesday, July 6, 2004 - 10:35 am:
Uhhh, not quite. Helium works not because it's inert, but because it's *insoluble*. Dissolved gases from the mobile phase diffuse into the helium bubble and are swept from the system, but very little helium diffuses out of the bubble into the mobile phase because of helium's low solubility.
![]()
![]()
![]()
![]()
![]() By Anonymous on Tuesday, July 6, 2004 - 10:50 am:
By Anonymous on Tuesday, July 6, 2004 - 10:50 am:
Thank u all for our comments
![]()
![]()
![]()
![]()
![]() By HW Mueller on Tuesday, July 6, 2004 - 11:52 pm:
By HW Mueller on Tuesday, July 6, 2004 - 11:52 pm:
Well, it turns out that He dissolves enough in H2O to raise the baseline in a UV detector almost as much as N2 in H2O (comparing to H2O degassed under vacuum). It just seems to have less degassing tendency.
It appears that I mentioned that before, maybe I should publish these exprimental results.
![]()
![]()
![]()
![]()
![]() By HW Mueller on Wednesday, July 7, 2004 - 12:18 am:
By HW Mueller on Wednesday, July 7, 2004 - 12:18 am:
Arghh, I meant "less outgassing tendency", above.
![]()
![]()
![]()
![]()
![]() By Anonymous on Wednesday, July 7, 2004 - 01:59 am:
By Anonymous on Wednesday, July 7, 2004 - 01:59 am:
Girls, in our lab, had tried to make me breath helium in and talk, while exhaling it. They said, my voice will be like that of cartoon creatures.
![]()
![]()
![]()
![]()
![]() By Alexander on Wednesday, July 7, 2004 - 05:51 pm:
By Alexander on Wednesday, July 7, 2004 - 05:51 pm:
As I've heard, He has an unusual solubility - temperature dependence. While other gases increasingly soluble at lower temperatures, He has the lowest solubility in cold water and higher solubility in hot water. So, when you cool the degassed buffer, the excessive He will actually eliminate, sort of “preserving” the buffer from dissolving N2/O2 again during a storage.
Can anyone confirm my statement or it’s a legend?
![]()
![]()
![]()
![]()
![]() By bill tindall on Friday, July 9, 2004 - 06:46 am:
By bill tindall on Friday, July 9, 2004 - 06:46 am:
Alexander,
First my compliments for providing a name. It encouraged me to look up the information to answer your question. According to data in Handbook of Chem and Phy. helium solubility has a relatively small temperature dependence over the practical LC range, but it is true that it is somewhat more soluble at 80 C than room temp. However the solubility also increases at temperatures less than room temperature, ie the solubility peaks at about room temperature. I would question whether this difference results in any practical benefit.
HW, before you publish you should check with your resident atomic spectroscopist to see if they agree with your hypothesis that helium at a mole fraction of about 10e-8 in a 1 cm cell will show measureable absorbance.
![]()
![]()
![]()
![]()
![]() By HW Mueller on Friday, July 9, 2004 - 08:04 am:
By HW Mueller on Friday, July 9, 2004 - 08:04 am:
Bill,
the rise in baseline due to He can, of course, not be due to an absorbance, it must be a scattering effect.
Do you know of a UV spectroscopist who works almost exclusively below 0.001 AU?
My peak hights are almost always below that. The rise in baseline due to saturation with N2, O2, or He have been in the neighborhood of 0.002 AU, compared with thoroughly evakuated H2O. If one does not see this ones pump and plumbing probably airates the mobile phase. If your system is well equilibrated this shift in baseline doesn´t matter, your UV detector should be zeroed anyway. But, some of the unexplained baseline drifts, shifts, and peaking/spiking (that sometimes bother HPLC) during runs are probably caused by changes in gas concentrations.
![]()
![]()
![]()
![]()
![]() By bill on Saturday, July 10, 2004 - 05:25 pm:
By bill on Saturday, July 10, 2004 - 05:25 pm:
HW,
ICP emmision spectroscopists work with extremely low level signals and path lengths of 1/2 to 1 meter and wavelengths well below 200 nm for some elements.
Spectometer optics are usually purged with either nitrogen or argon. Don't know anything about helium, but making the assumption that it is closer to argon than oxygen, which is significantly absorbing in the UV.
Given the mole fraction solubility of gases in water at a pressure of 760 mm is about 10e-5 ,an absorbance of 0.001 in water, if true, would translate to quite a loss of signal in an ICP spectrometer at a gas pressure of 1 atmosphere and a 1/2 to 1 meter path length, making the assumption that the absorbtivity in the gas phase is similar to the gas molecule dissolved in water.
A well equipped UV lab will have a long path gas cell in which the absorbance of gases can be measured, if person in the lab don't already know or can't find the data.
![]()
![]()
![]()
![]()
![]() By HW Mueller on Tuesday, July 20, 2004 - 02:22 am:
By HW Mueller on Tuesday, July 20, 2004 - 02:22 am:
Just peeking in from vacation. The stuff I mentioned was done at 220 nm, that´s why I assumed that absorption is not involved. Also, if one vigorously gasifies with He one sees spikes in addition to the raised baseline, indicating that tiny bubbles are involved (at +100 bar, if memory serves me correctly). These bubbles were not visible with the "naked" eye. The phenomenon was the same as that observed with air or N2, only slightly lower in intensity.
![]()
![]()
![]()
![]()
![]() By tom jupille on Tuesday, July 20, 2004 - 11:38 am:
By tom jupille on Tuesday, July 20, 2004 - 11:38 am:
The following is taken from:
http://www.faqs.org/faqs/sci/chem-faq/part5/section-5.html
I'ver reformatted the table slightly to (hopefully) display properly.
The following data is from Kaye and Laby, 13th edition, and the units are
the number of cm3 of gas at 0C and 760 mmHg which dissolve in 1 cm3 of water
at the temperature stated ( when the gas is at 760 mmHg pressure and in
equilibrium with the water ).
Temp.(C)...0.......10.......20.......30.......40.......50.......60
Helium---0.0098---0.0091---0.0086---0.0084---0.0084---0.0086---0.0090
Hydrogen-0.0214---0.0195---0.0182---0.0170---0.0164---0.0161---0.0160
Nitrogen-0.0230---0.0185---0.0152---0.0133---0.0119---0.0108---0.0100
Oxygen---0.047----0.037----0.030----0.026----0.022----0.020----0.019
Argon----0.054----0.041----0.032----0.028----0.025----0.024----0.023
CO2------1.676----1.163----0.848----0.652----0.518----0.424----0.360
I've no explanation for the aberrant trend for helium at higher temperatures,
but I assume it's real - but it's irrelevant for HPLC solvents that are
usually stored at ambient temperature. Points to note - the lower solubility
of helium over the range of concern, *and* the lower rate of change of
decreasing solubility with increasing temperature. There is heat generated
in the compression of the solvent, along with friction in HPLC pump heads
and, more importantly, HPLC columns are often heated - thus the solvent
could outgas and form bubbles in UV detector cells that are at ambient.
By using helium, there is less chance of that happening. For example, if the
temperature increased from 10C to 40C, the undissolved gas volume would be
0.0007 cm3 for helium, and 0.0066 cm3 for nitrogen.
![]()
![]()
![]()
![]()
![]() By HW Mueller on Monday, August 2, 2004 - 02:51 am:
By HW Mueller on Monday, August 2, 2004 - 02:51 am:
Just back from vacation.
Thanks Tom for the data. As mentioned above, I got outgassing (evidenced by spiking) only after very vigorous gassing with He or N2 . Otherwise there was a smooth but raised baseline with near sat. N2 or He, in comparison to extensively evacuated water. maybe the gas changes the refractive index? Actually, it is not really so surprising when one considers that a change in flow rate or a pressure wave also causes baseline changes (refractive index, scattering changes).
![]()
![]()
![]()
![]()
![]() By tom jupille on Monday, August 2, 2004 - 11:48 am:
By tom jupille on Monday, August 2, 2004 - 11:48 am:
I'dm inclined to agree with you that an RI shift is the likely culprit. I suppose hanging an RI detector on the end of the system would let you measure it directly.
The other reason for degassing is minimizing the formation of bubbles when solvents are mixed (solubility of air in mixed solvents is a non-linear function of solvent composition). In that respect, I was surprised that the difference in water solubility between He and N2 was so small (2X). It would be interesting to see a plot for helium similar to that for oxygen (the attached figure is scanned in from the Snyder & Dolan Troubleshooting book).
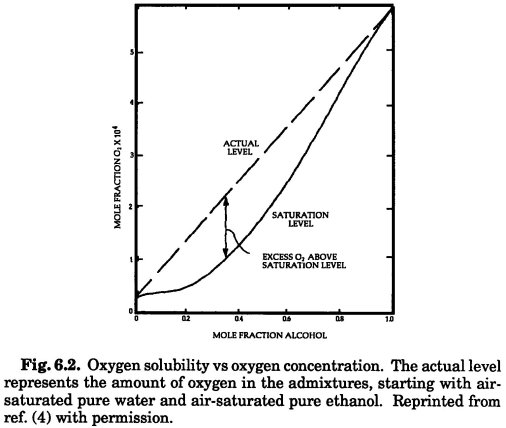
There's probably an academic thesis project in here somewhere!
![]()
![]()
![]()
![]()
![]() By HW Mueller on Monday, August 2, 2004 - 11:28 pm:
By HW Mueller on Monday, August 2, 2004 - 11:28 pm:
From the mentioned results I surmised that the He conc. must be considerable, but your figures also surprised me at first. The broad peaks one gets upon injecting small amounts of air (or from dissolved air in sample) must also be due to an RI effect, though sometimes I have seen foam emerge from the detector during such a peak.
Yes, gases in HPLC are quite interesting.
One should note that a rise in baseline due to gases is normally not seen if the gas is there from the start and if it has a constant concentration, as UV detectors are zeroed...